Click to read the article in Turkish
The İstanbul Metropolitan Municipality (İMM) Scientific Advisory Board has shared its assessment of Turkey's novel coronavirus (COVID-19) vaccination that started with health workers on January 13.
Before elaborating on the ongoing vaccination with the first dose of China-based Sinovac company's CoronaVac, the Scientific Board has underlined that at least 70 percent of the population must be vaccinated with effective and safe vaccines for immunization against the disease.
'Preliminary results still not shared with the public'
"Under normal circumstances, a vaccine's Phase-3 trials must end, the results must be published and they must be monitored afterwards in order to determine whether the vaccine is safe and effective and to implement it among wider society," the Board has underlined.
Referring to the emergency of vaccinating people against COVID-19, considering the high infection rates and numbers of cases and deaths, the Advisory Board has reminded the public that an emergency use authorization is now given for the vaccines without waiting for the completion of Phase-3 trials for these reasons and briefly added:
"As of January 18, the Phase-3 preliminary results of Pfizer-BioNTech, Moderna and Oxford-AstraZeneca vaccines have been announced.
"However, the Phase-3 preliminary results of the CoronaVac, still the only vaccine used by Turkey, have still not been presented to the public, even as a scientific report. On the contrary, different results from different countries as to this vaccine have been reported in the press, without citing the subgroup analyses. Similarly, the Phase-3 preliminary results of the CoronaVac vaccine in Turkey are not known, either."
Against this background, the Scientific Advisory Board has underlined the importance of transparency and scientific information in building public trust in vaccines, especially in the face of vaccine hesitancy and refusal.
"Within this context, the preliminary results of the CoronaVac's Phase-3 trials must be immediately reviewed by an independent scientific board and announced to the public," the Board has noted.
'Vaccination started 1 month late'
Sharing the rates of vaccinated populations in different countries so far, the İMM's Scientific Advisory Board has commented that while Turkey has started vaccinating its people rather late due to the delays in vaccine procurement, Turkey now ranks third in daily vaccination capacity, after the United State (US), China and the United Kingdom (UK).
"On the other side, vaccination could not be started as scheduled in Turkey and there was a delay of nearly a month," the Board has noted.
"According to the Health Ministry data, 575,166 new cases were reported and 7,348 people lost their lives in this period. Due to the mistakes in the vaccination strategy, Turkey has made a contract for a single type of vaccine in doses insufficient for now, instead of inking a contract for more than one type of vaccine and for sufficient amounts, which emerges as the biggest obstacle to Turkey's way out from the pandemic," the Board has warned.
"It is, without a doubt, a major problem that only a single type of vaccine will be used in Turkey," the Board has further underlined.
Stressing the importance of vaccine diversity to break the mutation chain and to prevent a social destruction to be caused by the outbreak, the İMM's Science Board has noted that this deficiency must be eliminated immediately, the vaccines with their Phase-3 preliminary results announced must be made available for people and the fate of the CoronaVac must be decided based on its Phase-3 preliminary results.
'Vaccines must be free, equally implemented'
Reminding the public that COVID-19 vaccines cannot fully prevent infections, the Science Board has underlined that personal protective measures such as masks, distance and hygiene are still very important.
Reiterating the importance of providing people with a sufficient number of vaccines and doses, vaccinating people quickly by prioritizing risk groups and sharing information about all processes of vaccination transparently, the İMM's Scientific Advisory Board has concluded that "within this context, the entire society must be vaccinated equally and free of charge by prioritizing effective and safe vaccines and risk groups as a principle".
Briefly about vaccination in Turkey so far
After health care professionals, Turkey started vaccinating residents and workers in care and nursing homes against COVID-19 yesterday.
People staying and working at official and private care homes, disabled care centers, and nursing homes under the ambit of Turkey's Family, Labor and Social Services Ministry received the first dose of the China-based Sinovac Biotech's novel coronavirus (COVID-19) vaccine.
People 90 and above, who are staying at their homes, are also among the second group of people to be vaccinated. The program is planned to be completed in a week with inoculation of a total of 87,120 people including 18,450 personnel, 30,000 disabled people in care homes, 14,470 personnel and 24,200 elderly people in the nursing homes.
Health Minister Fahrettin Koca said on January 18 that according to the schedule, the inoculation of health workers will be completed by today.
The first batch of 3 million doses of the vaccine developed by China's Sinovac arrived in Turkey on December 30, 2020.
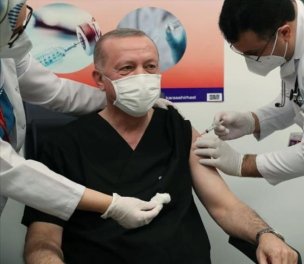
In addition to healthcare workers, President and ruling Justice and Development Party (AKP) Chair Recep Tayyip Erdogan and other top officials were also vaccinated in a stated attempt to help encourage the public. Health Minister Koca became the first person in the country to receive the vaccine jab after Turkey approved CoronaVac for emergency use. (SO/SD)







-132.jpg)