Click to read the article in Turkish
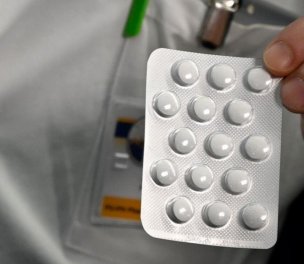
The Health Ministry's Pharmaceuticals and Medical Devices Agency (TİTCK) has made a statement about the allegations that the labels on the medicine with "favipiravir" active ingredient are changed and new labels are put on the expired medicine, which are then given to patients.
Referring to the related allegations expressed on social media, the TİTCK has said, "The allegations that expired medicine is given to coronavirus patients for treatment do not reflect the truth."
'Updating the dates is a routine practice'
The TİTCK has also stated that the applications for a license made to their Agency about the medicine with "favipiravir'' active ingredient indicated in the COVID-19 Guide published by the Public Health General Directorate are evaluated urgently and with priority.
Noting that "the stability studies on the related medicine takes a long time", the Agency has said that "updating the expiry dates depending on the results of these studies is a routine practice."
"Dates are updated within the knowledge and with the approval of our Agency and by putting a label on the medicine by the firm," it has added.
TTB warned against its use by children
The Turkish Medical Association (TTB) made a statement about the medicine used for the treatment of COVID-19 and objected to the use of favipiravir for children aged 12. Indicating that the Health Ministry does not offer a treatment in line with a scientific guide, the TTB said:
"Putting the use of Favipiravir for the treatment of children on the agenda without eliminating the concerns about its use for adults will do nothing but increase the concerns. We emphasize that we are afraid in the name of public health that a disaster similar to the 14-month hydroxychloroquine will unfold." (KÖ/SD)